Abstract
Background: Juvenile myelomonocytic leukemia (JMML) is an uncommon, frequently fatal clonal myeloproliferative neoplasm of early childhood driven by mutations in Ras pathway genes and characterized by hyperactive Ras/MAPK signaling. Clinical outcomes for many children with JMML remain dismal despite use of cytoreductive chemotherapy and hematopoietic stem cell transplantation, indicating continued need for new therapeutic approaches. Others and we have demonstrated promising preclinical activity of MEK inhibition in PTPN 11 -mutant (PTPN11) JMML, but the specific signaling characteristics and potential therapeutic vulnerabilities of the more recently-discovered CBL -mutant (CBL) JMML are not known. We hypothesized that genetic subtypes of JMML have distinct biochemical signaling profiles that may be differentially targeted with specific kinase inhibitors.
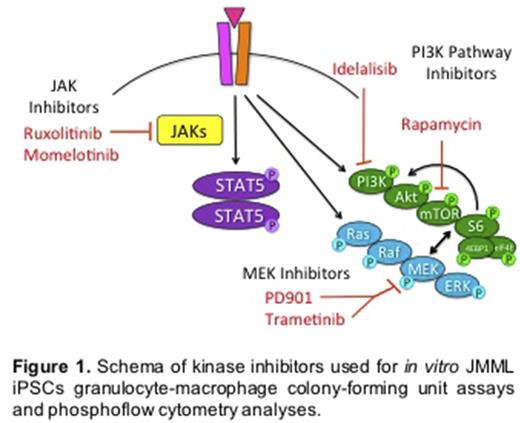
Methods: To test this hypothesis, we created induced pluripotent stem cell (iPSC) lines from children with JMML harboring somatic PTPN11E76K or germline CBLY371 mutations and from a normal healthy donor. We measured hypersensitivity of iPSCs to granulocyte-macrophage colony-stimulating factor (GM-CSF) in methylcellulose-based colony-forming unit assays (MCAs). We then assessed the ability of MEK, JAK, and PI3K pathway inhibitors (Figure 1) to inhibit colony formation and signaling phosphoproteins using MCAs and phosphoflow cytometry (PC) analysis, respectively.
Results: We observed similar in vitro GM-CSF hypersensitivity and colony formation of PTPN11 and CBL iPSCs in MCAs, recapitulating the phenotype of primary JMML cells. We detected constitutive Ras/MAPK (phosphorylated ERK; pERK) and PI3K pathway signaling (pS6 and pAkt) by PC analysis in both PTPN11 and CBL iPSCs versus control iPSCs (p<0.001 and p<0.05 by ANOVA, respectively) with greater pERK levels in PTPN11 cells (p<0.05 by Tukey's post-test). As predicted, activated signaling and colony formation of PTPN11 iPSCs were preferentially inhibited by the MEK inhibitors PD0325901 (PD901) and trametinib in PC analyses and in MCAs. Signaling and colony growth were abrogated in both JMML iPSC subtypes incubated with the PI3Kd inhibitor idelalisib or the mTOR inhibitor rapamycin. In contrast, we detected increased pJAK2 and pSTAT5 levels in CBL iPSCs (p<0.05), but not in PTPN11 iPSCs, with inhibition of colony growth and JAK/STAT signaling in CBL iPSCs incubated with the JAK inhibitors momelotinib and ruxolitinib. These data suggest preferential sensitivity of PTPN11 iPSCs to MEK inhibitors and of CBL iPSCs to JAK inhibitors, as well as similar sensitivity of both JMML iPSC subtypes to PI3K and mTOR inhibition.
Conclusions: Our studies demonstrate mutation-specific signaling profiles of JMML iPSCs and therapeutic potential of Ras/MAPK, JAK/STAT, and PI3K/Akt/mTOR pathway kinase inhibitors in genetic subsets of JMML. Creation of additional iPSCs from patients with other JMML-associated mutations will facilitate assessment of additional drugs, alone or as combination therapy. Clinical investigation of mutation-specific kinase inhibitor therapies in children with JMML is warranted.
Tasian: Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead Sciences, Inc.: Research Funding. Gandre-Babbe: GlaxoSmithKline: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal